Spectroscopy checks Battery Capacity in 15 Seconds
Getting hold of the elusive battery
Battery test methods are failing and the industry demands better technologies. Without suitable tools, performance assessment remains guesswork, resulting in replacing batteries too soon or too late (mostly too late). Some batteries are swapped repeatedly without knowing the cause of failure, other are changed on a date stamp not knowing the condition, but most are left untouched until a breakdown occurs.
There are no shortages of battery testers. Most claim to measure state-of-health (SoH) by reading voltage and internal resistance. Batteries have improved and resistance measurement alone is ill-suited to estimate performance. Better electrolytes and corrosion-resistant materials keep the resistance low throughout battery life. Advertising features that lay outside the unit’s capability is misleading and confuses the industry into believing that multifaceted results are attainable with basic test methods.
Most battery testers are capable of identifying a dying or dead battery, however; but so does the user. The challenge comes in assessing a battery before the performance is compromised. This is done by measuring the capacity, the leading health indicator of a battery. The most reliable method is through a full charge/discharge, but this requires removing the battery from service. Although suitable for smaller packs, discharges are impractical for larger batteries. A lead acid loses about 2% of its capacity with each full cycle and a starter battery as much as 8%. This raises the need for advanced rapid-testing as a battery test without assessing capacity is incomplete.
Figure 1 illustrates the three components of a battery consisting of the stored energy (capacity), the empty portion that can be refilled and the inactive part that is lost due to usage and aging. Rated capacity refers to the manufacturer’s specified capacity in Ah (ampere-hours), a value that only applies while the battery is new. The available capacity is the energy storage capability after a full charge, and State-of-charge (SoC) refers to the stored energy that also includes the inactive part. This causes inaccuracies in fuel gauging as a fully charged battery will always reads 100% even if 50% of the battery capacity has become inactive. The runtime of such a battery is cut to 50%.
Battery testing has been around since the battery was invented. A simple but reliable rapid-test method is the carbon pile that applies a brief load to check current and voltage stability. A skilled mechanic can assess a battery on its kinetic behaviors, but capacity estimation is not possible.
A further unknown is SoC. This can be estimated by measuring the open terminal voltage of a battery that has rested for a few hours and resides at room temperature. Simply opening the car door or cranking the engine disturbs the tranquility and neutralization takes several hours; the manufacturer recommends 24h. (There are similarities with an agitated spouse.) Knowing the state-of-charge is important since a battery with a low charge behaves similarly to one with a faded capacity. These two characteristics must be checked separately when testing a battery.
AC conductance meter, also known as impedance tester, has mostly replaced the carbon pile. Small and non-invasive, the impedance tester injects an AC signal to measure the internal battery resistance. These units check the CCA (cold cranking amp) of starter batteries and the resistance of UPS batteries; capacity estimation is outside their capabilities.
Critical progress has been made with electrochemical impedance spectroscopy (EIS). EIS is not new; high cost, long test times and the need for trained staff to interpret reams of data restricted the technology to laboratories.
Cadex took EIS one step further and developed multi-model electrochemical impedance spectroscopy, or Spectro™. A frequency scan produces a Nyquist plot onto which various electrochemical models are superimposed. Data fusion correlates the values to derive at capacity reading. (CCA and SoC use more simplistic test methods.) The test takes 15 seconds and the battery must have a minimal charge of 60%. Best results are attained with a “working” battery. New batteries that lack formatting or packs that have been in long storage do not exhibit the same symptoms than those pulled from service.
A starter battery is declared functional if it can crank the engine. With modern vehicles, this is not the only criteria and the driver asks: “Can a steer and break?” Auxiliary functions, including start-stop, require good capacity reserve, pushing capacity estimations to the forefront. Characterization of the test results is another reason for the gravitation towards capacity. CCA does not represent the battery state-of-health well; the readings stay stable with age while capacity fades predictably. Capacity estimations, however, requires more complex technology than CCA reading, using matrices as look-up tables.
Figure 2 illustrates the behavior of 20 aging starter batteries sorted from good to bad. All packs cranked well but unknown to the users batteries 10 to 20 were at the end of life. A battery with less than 40% capacity should be replaced as it can get the driver stranded, especially during a cold snap. Capacity fade goes mostly unnoticed; an analogy is a horse that gallops until it drops dead of exhaustion.
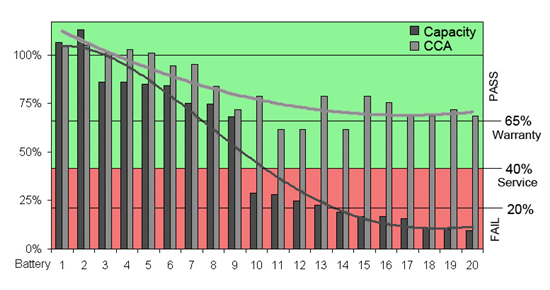
Figure 2: Capacity and CCA of aging batteries. Batteries 1–9 have good CCA and high capacities; batteries 10–20 are at the end-of-life with capacity loss. All batteries crank well. Test Method CCA was taken with Spectro CA-12; capacity was measured with an Agilent load bank by applying full discharges according to BCI standards.
Capacity loss of a lead acid battery is triggered by corrosion, sulfation and the shedding of active material, each causing the internal resistance to rise. While this is true, tests reveal that the resistance on most starter batteries stays low while capacity declines. To explore the relationship further, Cadex tested 175 aging starter batteries in a extensive test and found startling results. The correlation between capacity and resistance (or CCA) was only 0.55; 1 would present a perfect match.
Figure 3 demonstrates these findings graphically. The vertical Y-axis shows CCA readings; the horizontal X-axis represents capacities. The diamonds do not hug the red reference line as they would with a close correlation between capacity and CCA. Instead, borderline batteries gravitate towards the 40% capacity line on the left green field; very few hug the low 50% CCA threshold line.
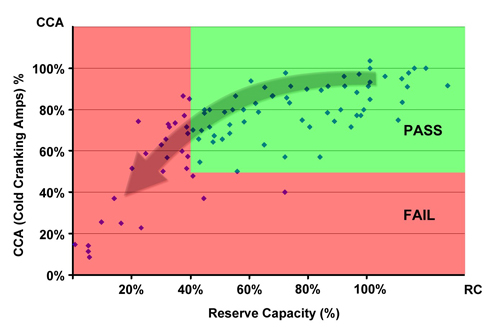
Figure 3: Relationship between CCA and capacity on 175 starter batteries The green field represents PASS; batteries in the red FAIL field have reached the end-of-life. Most batteries hug the 40% capacity line on the left the green field; few touch the 50% CCA line on the bottom. Test method Capacity and CCA are tested according to SAE J537
While corrosion and the shedding of active material are irreversible, sulfation can be corrected if caught in time. This is done by applying an elevated charge voltages or current pulses for a time to dissolve the lead-sulfates that form when a battery remains under-charged. A sulfation detection method are need that applies to right amount of antidote as not to harm the battery by over correction. Insufficient charge occurs in city driving with auxiliary loads engaged; idling or driving at low speed cannot fully charge a battery.
Battery diagnostics by rapid-testing is still in its infancy. We don’t even have a reliable method to measure state-of-charge, let alone getting a reliable capacity measurement on the fly. Batteries cannot be measured per se; their health can only be predicted with multiple measurements similar to a weather forecasting or a medical examination.
Scientists place high hopes in electrochemical impedance spectroscopy, a technology that will also improve BMS (battery management system). Capacity is the leading health indicator and adding this dimension to a BMS will greatly improve end-of-life prediction. Capacity has been the missing link for too long and the elusive battery is finally giving its true color.
Battery test methods are failing and the industry demands better technologies. Without suitable tools, performance assessment remains guesswork, resulting in replacing batteries too soon or too late (mostly too late). Some batteries are swapped repeatedly without knowing the cause of failure, other are changed on a date stamp not knowing the condition, but most are left untouched until a breakdown occurs.
There are no shortages of battery testers. Most claim to measure state-of-health (SoH) by reading voltage and internal resistance. Batteries have improved and resistance measurement alone is ill-suited to estimate performance. Better electrolytes and corrosion-resistant materials keep the resistance low throughout battery life. Advertising features that lay outside the unit’s capability is misleading and confuses the industry into believing that multifaceted results are attainable with basic test methods.
Most battery testers are capable of identifying a dying or dead battery, however; but so does the user. The challenge comes in assessing a battery before the performance is compromised. This is done by measuring the capacity, the leading health indicator of a battery. The most reliable method is through a full charge/discharge, but this requires removing the battery from service. Although suitable for smaller packs, discharges are impractical for larger batteries. A lead acid loses about 2% of its capacity with each full cycle and a starter battery as much as 8%. This raises the need for advanced rapid-testing as a battery test without assessing capacity is incomplete.
Figure 1 illustrates the three components of a battery consisting of the stored energy (capacity), the empty portion that can be refilled and the inactive part that is lost due to usage and aging. Rated capacity refers to the manufacturer’s specified capacity in Ah (ampere-hours), a value that only applies while the battery is new. The available capacity is the energy storage capability after a full charge, and State-of-charge (SoC) refers to the stored energy that also includes the inactive part. This causes inaccuracies in fuel gauging as a fully charged battery will always reads 100% even if 50% of the battery capacity has become inactive. The runtime of such a battery is cut to 50%.
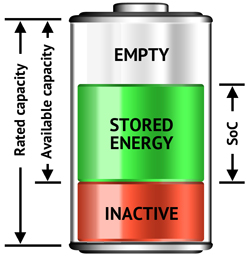 |
Figure 1: Three parts of a battery A battery consists of stored energy, the empty portion that can be recharged and the inactive that is permanently lost due to aging. |
Testing Starter Batteries
Battery testing has been around since the battery was invented. A simple but reliable rapid-test method is the carbon pile that applies a brief load to check current and voltage stability. A skilled mechanic can assess a battery on its kinetic behaviors, but capacity estimation is not possible.A further unknown is SoC. This can be estimated by measuring the open terminal voltage of a battery that has rested for a few hours and resides at room temperature. Simply opening the car door or cranking the engine disturbs the tranquility and neutralization takes several hours; the manufacturer recommends 24h. (There are similarities with an agitated spouse.) Knowing the state-of-charge is important since a battery with a low charge behaves similarly to one with a faded capacity. These two characteristics must be checked separately when testing a battery.
AC conductance meter, also known as impedance tester, has mostly replaced the carbon pile. Small and non-invasive, the impedance tester injects an AC signal to measure the internal battery resistance. These units check the CCA (cold cranking amp) of starter batteries and the resistance of UPS batteries; capacity estimation is outside their capabilities.
Critical progress has been made with electrochemical impedance spectroscopy (EIS). EIS is not new; high cost, long test times and the need for trained staff to interpret reams of data restricted the technology to laboratories.
Cadex took EIS one step further and developed multi-model electrochemical impedance spectroscopy, or Spectro™. A frequency scan produces a Nyquist plot onto which various electrochemical models are superimposed. Data fusion correlates the values to derive at capacity reading. (CCA and SoC use more simplistic test methods.) The test takes 15 seconds and the battery must have a minimal charge of 60%. Best results are attained with a “working” battery. New batteries that lack formatting or packs that have been in long storage do not exhibit the same symptoms than those pulled from service.
A starter battery is declared functional if it can crank the engine. With modern vehicles, this is not the only criteria and the driver asks: “Can a steer and break?” Auxiliary functions, including start-stop, require good capacity reserve, pushing capacity estimations to the forefront. Characterization of the test results is another reason for the gravitation towards capacity. CCA does not represent the battery state-of-health well; the readings stay stable with age while capacity fades predictably. Capacity estimations, however, requires more complex technology than CCA reading, using matrices as look-up tables.
Figure 2 illustrates the behavior of 20 aging starter batteries sorted from good to bad. All packs cranked well but unknown to the users batteries 10 to 20 were at the end of life. A battery with less than 40% capacity should be replaced as it can get the driver stranded, especially during a cold snap. Capacity fade goes mostly unnoticed; an analogy is a horse that gallops until it drops dead of exhaustion.

Figure 2: Capacity and CCA of aging batteries. Batteries 1–9 have good CCA and high capacities; batteries 10–20 are at the end-of-life with capacity loss. All batteries crank well. Test Method CCA was taken with Spectro CA-12; capacity was measured with an Agilent load bank by applying full discharges according to BCI standards.
Capacity loss of a lead acid battery is triggered by corrosion, sulfation and the shedding of active material, each causing the internal resistance to rise. While this is true, tests reveal that the resistance on most starter batteries stays low while capacity declines. To explore the relationship further, Cadex tested 175 aging starter batteries in a extensive test and found startling results. The correlation between capacity and resistance (or CCA) was only 0.55; 1 would present a perfect match.
Figure 3 demonstrates these findings graphically. The vertical Y-axis shows CCA readings; the horizontal X-axis represents capacities. The diamonds do not hug the red reference line as they would with a close correlation between capacity and CCA. Instead, borderline batteries gravitate towards the 40% capacity line on the left green field; very few hug the low 50% CCA threshold line.

Figure 3: Relationship between CCA and capacity on 175 starter batteries The green field represents PASS; batteries in the red FAIL field have reached the end-of-life. Most batteries hug the 40% capacity line on the left the green field; few touch the 50% CCA line on the bottom. Test method Capacity and CCA are tested according to SAE J537
While corrosion and the shedding of active material are irreversible, sulfation can be corrected if caught in time. This is done by applying an elevated charge voltages or current pulses for a time to dissolve the lead-sulfates that form when a battery remains under-charged. A sulfation detection method are need that applies to right amount of antidote as not to harm the battery by over correction. Insufficient charge occurs in city driving with auxiliary loads engaged; idling or driving at low speed cannot fully charge a battery.
Summary
Battery diagnostics by rapid-testing is still in its infancy. We don’t even have a reliable method to measure state-of-charge, let alone getting a reliable capacity measurement on the fly. Batteries cannot be measured per se; their health can only be predicted with multiple measurements similar to a weather forecasting or a medical examination.Scientists place high hopes in electrochemical impedance spectroscopy, a technology that will also improve BMS (battery management system). Capacity is the leading health indicator and adding this dimension to a BMS will greatly improve end-of-life prediction. Capacity has been the missing link for too long and the elusive battery is finally giving its true color.